Market and Competitive Opportunity Solution help a Medical Device Company Assess the Landscape of Cell Imaging Technology domain| Identification of Top Competitors through Competitive Intelligence | DelveInsight
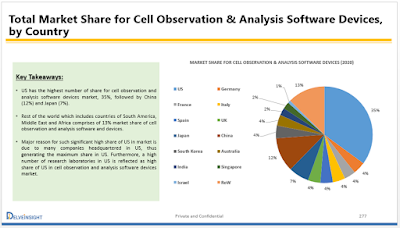
(Albany, US) Traditional method of toxicity and drug safety studies is expensive, has a low success rate, and is resource- and time-consuming. To overcome these challenges, pharmaceutical companies are increasingly adopting high-content screening (HCS) cell-based assays for identifying the effects of toxicity in drug development studies (cell-based imaging enables the monitoring of drug toxicity mechanisms, such as oxidative stress, micronuclei, mitochondrial dysfunction, steatosis, and apoptosis). DelveInsight is the world's leading independent provider of strategic market intelligence solutions. Our market intelligence services are designed to connect your organization’s goals with global opportunities. RFP to know more about DelveInsight’s competitive intelligence for pharma companies . Market & Competitive Assessment Business Problem: A large Asian conglomerate was working to develop their asset portfolio in Cell Imaging and Analysis, and was interested to assess t