COVID-19 Competitive landscape Report 2022 by DelveInsight
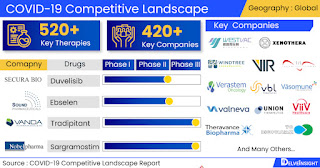
COVID-19 pipeline constitutes 420+ key companies continuously working towards developing 520+ COVID-19 treatment therapies, analyzes DelveInsight
COVID-19 is the result of infection with severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first isolated
and identified in patients who were exposed at a seafood market in Wuhan City,
Hubei Province, China in December 2019. Similar to findings related to SARS-CoV
and Middle East respiratory syndrome coronavirus, SARS-CoV-2 is believed to
cross species to initiate primary human infections; it is now spread primarily
by human-to-human transmission.
DelveInsights, “COVID-19 Competitive landscape, 2022” report provides comprehensive insights about 420+ companies and COVID-19 drugs in COVID-19 Competitive
landscape. It covers the therapeutics assessment by product type, stage, route of administration, and molecule type.
It further highlights the inactive pipeline products in this space.
To know more about
the COVID-19 Competitive landscape report, click here: https://www.delveinsight.com/report-store/covid-19-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Takeaways from
the COVID-19 Competitive landscape report:
- Leading
COVID-19 Companies
working in the market are Westvac Biopharma, Xenothera, Windtree
Therapeutics, Vir Biotechnology, ViiV Healthcare, Verastem, VBL
Therapeutics, Valneva, Trustem, Toscana Life Sciences, Throne
Biotechnologies, Thirty respiratory Limited, Theravance Biopharma, TC
BioPharm, Syntax for Science,S.L, SyneuRx, Swedish Orphan Biovitrum,
Swedish Herbal Institute AB, Stemedica Cell Technologies, Sound
Pharmaceuticals, Sorrento Therapeutics and Others.
- Key COVID-19 therapies in various stages of development
include SKYCovione, Sotrovimab, Sargramostim, Tradipitant, XAV-19,
MAD0004J08, RESP301, Niclosamide, Sinapultide, MAS-825, Maraviroc,
Abivertinib, Ebselen, Duvelisib, TCB008 and Others.
- In
April 2022, Direct Biologics, announced that the US Food and Drug
Administration has awarded their EV drug product ExoFlo with a
regenerative Medicine Advanced Therapy designation for the treatment of
Acute Respiratory Distress Syndrome associated with COVID-19.
DelveInsight’s COVID-19 Report covers around 520+ products
under different phases of clinical development like
- Assessment
by COVID-19
Product Type
- Assessment
by Stage and Product Type of COVID-19
- Assessment
by Route of Administration
- Assessment
by Stage and Route of Administration of COVID-19
- Assessment
by COVID-19
Molecule Type
- Assessment
by Stage and Molecule Type of COVID-19
Emerging COVID-19 Drugs Under Different Phases of
Clinical Development Include:
Some of the COVID-19 therapies are Sotrovimab, SKYCovione
and Many Others.
Further COVID-19 product details are provided in the
report. Download the COVID-19 Pipeline report to learn more about the emerging COVID-19 therapies at: https://www.delveinsight.com/sample-request/covid-19-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key companies in the
COVID-19 Therapeutics Market:
Some of the COVID-19
Companies working in the market are Westvac Biopharma, Xenothera, Windtree
Therapeutics, Vir Biotechnology, ViiV Healthcare, Verastem, VBL Therapeutics,
Valneva, Trustem, Toscana Life Sciences, Throne Biotechnologies, Thirty
respiratory Limited, Theravance Biopharma, TC BioPharm, Syntax for Science,S.L,
SyneuRx, Swedish Orphan Biovitrum, Swedish Herbal Institute AB, Stemedica Cell
Technologies, Sound Pharmaceuticals, Sorrento Therapeutics and Others.
Request for Sample
PDF Report to know in detail about the recent developments and
advancements in COVID-19 clinical trials– https://www.delveinsight.com/sample-request/covid-19-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Table of Content
(TOC)
1. COVID-19 Pipeline Report Introduction
2. COVID-19 Pipeline Report Executive Summary
3. COVID-19 Pipeline: Overview
4. Analytical
Perspective In-depth Commercial Assessment
5. COVID-19 Clinical Trial Therapeutics
6. COVID-19 Pipeline: Late Stage Products
(Pre-registration)
7. COVID-19 Pipeline: Late Stage Products (Phase
III)
8. COVID-19 Pipeline: Mid Stage Products (Phase
II)
9. COVID-19 Pipeline: Early Stage Products (Phase
I)
10. COVID-19 Pipeline Therapeutic Assessment
11. Inactive Products
in the COVID-19 Pipeline
12.
Company-University Collaborations (Licensing/Partnering) Analysis
13. Key COVID-19 Companies
14. Key Products in
the COVID-19 Pipeline
15. Unmet Needs
16. COVID-19 Market Drivers and Barriers
17. Future
Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF
Report to know more about @ https://www.delveinsight.com/sample-request/covid-19-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
About DelveInsight
DelveInsight is a
leading Business Consultant, and Market Research Firm focused exclusively on
life sciences. It supports pharma companies by providing comprehensive
end-to-end solutions to improve their performance.
Comments
Post a Comment