Gene Therapy Competitive landscape Report 2022 by DelveInsight
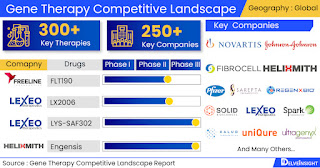
Gene Therapy pipeline constitutes 250+ key companies continuously working towards Developing 300+ Gene Therapy treatment therapies, analyses DelveInsight
Gene therapy is a technique that uses a gene(s) to
treat, prevent or cure a disease or medical disorder. Often, gene therapy works
by adding new copies of a gene that is broken, or by replacing a defective or
missing gene in a patient’s cells with a healthy version of that gene. Both
inherited genetic diseases (e.g. hemophilia and sickle cell disease) and
acquired disorders (e.g. leukemia) have been treated with gene therapy.
DelveInsight’s, “Gene
Therapy Competitive landscape, 2022,” report provides comprehensive insights about 250+ companies and Gene Therapy drugs in Gene Therapy Competitive landscape. It covers the therapeutics
assessment by product type, stage, route of
administration, and molecule type. It further highlights the inactive pipeline products in this space.
To know more about
the Gene Therapy Competitive landscape report, click here: https://www.delveinsight.com/report-store/gene-therapy-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Takeaways from
the Gene Therapy Competitive landscape report:
·
Leading Gene Therapy Companies working in the market are Novartis, Johnson&Johnson,
Fibrocell Technologies, Pfizer, Sarepta Therapeutics, REGENXBIO, Solid
Biosciences Inc., Lexeo Therapeutics, Spark Therapeutics, Xalud Therapeutics,
uniQure, Ultragenyx Pharmaceutical, Nanoscope Therapeutics, Homology Medicines,
Freeline Therapeutics, Amicus Therapeutics, CSL, Gyroscope Therapeutics,
Sangamo Therapeutics, Spark therapeutics, LYSOGENE, Asklepios
BioPharmaceutical, Alcyone Therapeutics, Taysha Gene Therapies, Gensight
Biologic and Others.
·
Key Gene Therapy therapies in various stages of development include Etranacogene
dezaparvovec, Engensis, Fordadistrogene movaparvovec, RGX-121, LYS-SAF302,
GT005, SPK 3006, FLT190, LX2006 and Others.
·
In March 2022, REGENXBIO, announced that the US Food and Drug
Administration granted Rare Pediatric Disease Designation for RGX-202, which
may entitle REGENXBIO to receive a priority review voucher which can be
redeemed to obtain priority review for any subsequent marketing application and
may be sold or transferred should a new BLA for RGX-202 be approved.
DelveInsight’s Gene Therapy Report covers around 300+ products
under different phases of clinical
development like
·
Assessment by Gene Therapy Product Type
·
Assessment by Stage and Product Type of Gene Therapy
·
Assessment by Route of Administration
·
Assessment by Stage and Route of Administration of Gene Therapy
·
Assessment by Gene Therapy Molecule Type
·
Assessment by Stage and Molecule Type of Gene Therapy
Emerging Gene Therapy Drugs Under Different Phases of
Clinical Development Include:
Some of the Gene Therapy therapies are bluebird bio Zynteglo,
RGX-121 and Many Others.
Further Gene Therapy product details are provided in the
report. Download the
Gene Therapy Pipeline report to learn more about the emerging Gene Therapy therapies at: https://www.delveinsight.com/sample-request/gene-therapy-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key companies in the
Gene Therapy Therapeutics Market:
Some of the Gene
Therapy Companies working in the market are Novartis, Johnson &Johnson,
Fibrocell Technologies, Pfizer, Sarepta Therapeutics, REGENXBIO, Solid
Biosciences Inc., Lexeo Therapeutics, Spark Therapeutics, Xalud Therapeutics,
uniQure, Ultragenyx Pharmaceutical, Nanoscope Therapeutics, Homology Medicines,
Freeline therapeutics, Amicus Therapeutics, CSL, Gyroscope Therapeutics,
Sangamo Therapeutics, Spark therapeutics, LYSOGENE, Asklepios
BioPharmaceutical, Alcyone Therapeutics, Taysha Gene Therapies, Gensight
Biologic and Others.
Request for Sample
PDF Report to know in detail about the recent developments and advancements in Gene Therapy clinical trials: https://www.delveinsight.com/sample-request/gene-therapy-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Table of Content
(TOC)
1. Gene Therapy Pipeline Report Introduction
2. Gene Therapy Pipeline Report Executive Summary
3. Gene Therapy Pipeline: Overview
4. Analytical
Perspective In-depth Commercial Assessment
5. Gene Therapy Clinical Trial Therapeutics
6. Gene Therapy Pipeline: Late Stage Products
(Pre-registration)
7. Gene Therapy Pipeline: Late Stage Products (Phase
III)
8. Gene Therapy Pipeline: Mid Stage Products (Phase
II)
9. Gene Therapy Pipeline: Early Stage Products (Phase
I)
10. Gene Therapy Pipeline Therapeutic Assessment
11. Inactive Products
in the Gene Therapy Pipeline
12.
Company-University Collaborations (Licensing/Partnering) Analysis
13. Key Gene Therapy Companies
14. Key Products in
the Gene Therapy Pipeline
15. Unmet Needs
16. Gene Therapy Market Drivers and Barriers
17. Future
Perspectives and Conclusion
18. Analyst Views
19. Appendix
About DelveInsight
DelveInsight is a
leading Business Consultant, and Market Research Firm focused exclusively on life
sciences. It supports pharma companies by providing comprehensive end-to-end
solutions to improve their performance.
Comments
Post a Comment